Lowest Cost Highest Efficiency Green Hydrogen
Advanced Renewable
Tue , 23 Apr 2024 21:59 WIB
The green hydrogen economy could be here already after the two obstacles faced so far have been resolved, namely production and logistics constraints. Production constraints so far are because the majority of hydrogen is still produced from methane reforming, which makes it dependent on fossils.
Hydrogen production using water electrolysis is constrained by energy requirements that are still greater than the energy content of hydrogen itself. On the logistics side, storing and transporting hydrogen is a challenge in itself, it requires a very high pressure of 700 Bar or a very low temperature of minus 253 degrees Celsius.
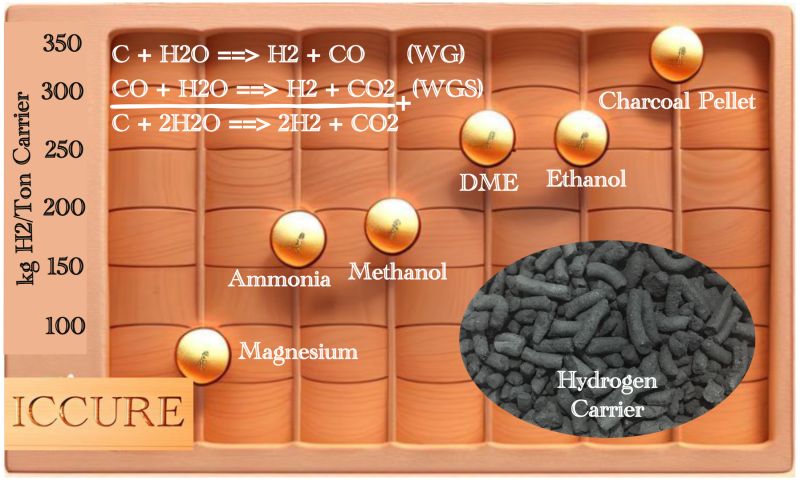
Some efforts have been done on logistics side, like using magnesium as a hydrogen carrier, for example - but apart from the expensive price of magnesium (US$ 6-8/kg), it is also only able to deliver around 7.7% of the weight of the carrier itself. Ammonia is better in terms of price (US$ 0.28/kg), but its H2 delivery capability is still low at around 17.6% of the ammonia weight, and still requires a pressure of 17 Bar for logistics.
A more interesting solution is to use oxygenates such as methanol, DME and Ethanol for the H2 carrier. Methanol at a price of US$ 0.33/kg can deliver H2 up to 18.75% of the weight of methanol, namely 12.5% from the H2 in the methanol molecule and an additional 6.25% from the steam used for reforming.
With the same pattern, DME and ethanol are capable of delivering hydrogen up to 26% of their respective weights, half of which is carried in the molecules and the other half is from the steam used for reforming. The prices of DME and ethanol are also relatively low, in the range of US$ 0.4/kg for DME and US$ 0.8/kg for ethanol.
Above all, it turns out that what is most interesting - at least from the results of our research, is a material that is very easy to produce, store and transport, namely charcoal. The price of charcoal is only US$ 0.2/kg and is capable of delivering 33.33% H2 of the charcoal weight. The reactions formula below explains this, every 1 C atom is capable of delivering 2 H2 molecules or 4 H atoms.
To convert charcoal into H2 is also easy, it only involves two main reactions, namely Water Gas (WG) to convert C into CO and 1 H2 molecule, then followed by the Water Gas Shift (WGS) reaction to convert the CO molecule into 1 more H2 molecule and CO2 as waste. These entire reactions can be carried out in one reactor, the Regenerative Energy Reactor, or through 2 reactors OCCYRE and Membrane reactor, I have uploaded all of these reactors before.
Because charcoal can be produced from any biomass, garbage and any waste, this means that green hydrogen can also be provided wherever you need it, from resources that are available around your location!
Other Post
Hilirisasi Sampah dan Limbah
Apr 23, 2024
Net-Zero Fuels and Power Generation
Apr 23, 2024
Categories
Renewable Energy






Please register first!
For post a new comment. You need to login first. Login
Comments
No comments